 |
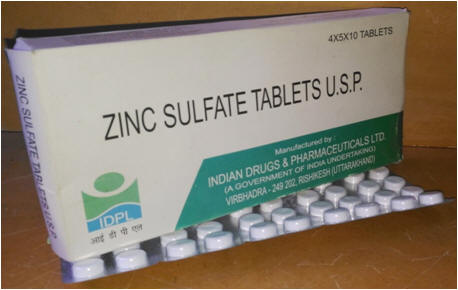
Zinc Dispersible tablets: (WHO & UNICEF have recommended use of zinc supplements in addition of ORS based dehydration therapy for management diarrhoea in children. Zinc supplements were added in the list of essential medicines provided by WHO in 2005. Estimate children mortality due to diarrhoea in India in the year 2004 was about 5,35,000. WHO recommends administration of zinc in the form of taste masked dispersible tablet of 20 mg strength. The technology for the same is challenging because of the bitter metallic after taste of zinc and intellectual property protection already taken by international companies. Dr. Arvind K. Bansal's group at NIPER took up this challenge & developed patented technology for manufacturing of zinc sulphate taste masked dispersible tablets. This technology was transferred to Indian Drugs and Pharmaceuticals Limited. |
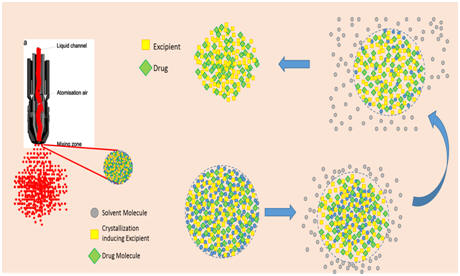 |
NanoCrySPTM Dr. Arvind K. Bansal's group has developed a novel 'bottoms-up' platform technology for the generation of nanocrystalline solid dispersions. They have demonstrated the biopharmaceutical benefits of this technology. Their group has also established the contribution of molecular mobility, heterogeneous nucleation, and the effect of excipients on nucleation and crystal growth, in the formation of nanocrystalline solid dispersions. |
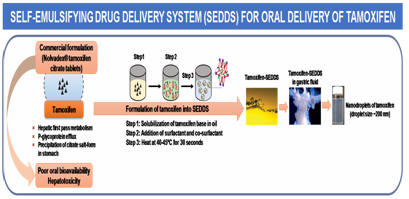 |
Tamoxifen-SEDDS Technology: Tamoxifen-SEDDS technology for effective treatment of breast cancer has been licensed to VAV Life Sciences, Mumbai for further development and commercialization. |
औषधि विभाग, रसायन और उर्वरक मंत्रालय,
भारत सरकार
Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, Government of India
Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, Government of India



